I’m still not a doctor. And I’m still learning new things every day – just like you – about this virus. These posts are a cultivation of information from official sources. After that, I try to break down the new information into digestible English as best I can.
The latest statistics
Here are a few numbers from some of the reputable sites collecting and releasing data….
CDC & WHO Numbers
Total Worldwide Cases: 372,757
Total Worldwide Deaths: 16,231
Total United States cases: 44,183
— Travel-related: 479
— Close contact: 569
— Under investigation: 43,135
Total deaths: 544
States reporting cases: 50 states, District of Columbia, Puerto Rico, Guam, and US Virgin Islands

COVID-19 cases reported by other medical sites:
Johns Hopkins
Total Worldwide Cases: 422,915
Total US cases: 55,148
Total US deaths: 796
Total US recovered: 349

Brian McNoldy, Senior Research Associate at Univ. of Miami’s Rosenstiel School, put that chart together. As it notes, it is pulling numbers from the Johns Hopkins totals. It shows the double-time of cases as about two-and-a-half days.
An examination of the ‘case fatality rate’ in China
A new research paper that is due out in June, that is not-yet-peer-reviewed, titled “Estimating Risk for Death from 2019 Novel Coronavirus Disease, China, January–February 2020” that looks into the mortality rate in China. And it may, indirectly, give some hints as to why the mortality rate in the United States has been so low so far.
Real quick, since this is a statistics-based research paper, I tend to give it a little more credence before peer-review than, say, a clinical trial. But since it is not-yet peer reviewed, know that it still need to go through the scientific hoops.
Alright, the authors point out in the paper that as the virus spread away from Wuhan, the likelihood of death decreased.
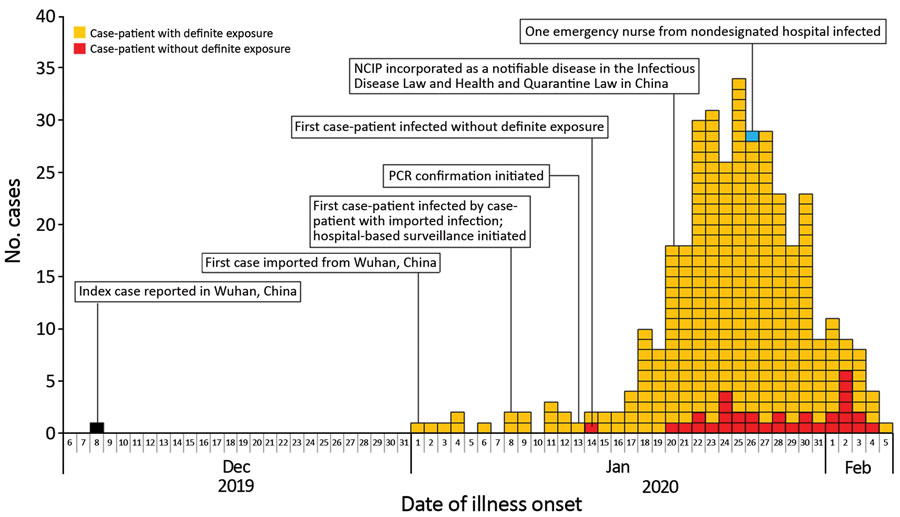
The authors of the paper suggest that the case-fatality rates, CFR, were “12.2% (95% CrI 11.3%–13.1%) in Wuhan, 4.2% (95% CrI 3.7%–4.7%) in Hubei Province excluding Wuhan, and 0.9% (95% CrI 0.7%–1.1%) in China excluding Hubei Province. The observed crude CFR was 4.2% (95% CI 3.9%–4.5%) in Wuhan, 1.8% (95% CI 1.6%–2.0%) in Hubei Province excluding Wuhan, and 0.43% (95% CI 0.32%–0.57%) in China excluding Hubei Province.”
That led them to conclude:
… the risk for death from COVID-19 in China as of February 11, 2020, were as high as 12% in the epicenter of the epidemic and as low as ≈1% in the less severely affected areas in China. Because the risk for death from COVID-19 is probably associated with a breakdown of the healthcare system in the absence of pharmaceutical interventions (i.e., vaccination and antiviral drugs), enhanced public health interventions (including social distancing measures, quarantine, enhanced infection control in healthcare settings, and movement restrictions), as well as enhanced hygienic measures in the general population and an increase in healthcare system capacity, should be implemented to rapidly contain the epidemic.
What does that mean for the United States? I would argue a lot.
Many people have been questioning why the death rate from COVID-19 has been so low in the United States so far. Are we healthier? Better equipped? More resilient? This paper may, indirectly, suggest that the reason the mortality rate has been low so far is that our hospitals have been situated to handle the extra cases – so far.
There are starting to be reports of hospitals in different parts of the country beginning to reach their threshold, though. And we are still toward the “beginning” of the spread.
And other hospitals can’t handle this type of exponential growth forever, either. That is why the last few sentences, where the authors highlight the need for social distancing and quarantine, are so important. They concluded that it wasn’t that the severity of the virus was worse in Wuhan, but rather that the severity of the symptoms were not able to be treated due to the overload on the medical community. So some died as a result of the lack of treatment.
So, in the United States, when the CDC urges people to wash hands and practice social distancing, this is what they are hoping to guard against. An overloaded medical system where doctors have to make tough decisions about who lives and who dies because they can only treat one person, not both.
From the CDC
The CDC released preliminary numbers for the infections so far. In the report, as of March 16, 4,226 COVID-19 cases had been reported in the United States. That number is now, three days later, 10,442. The report notes that news cases are now popping up at 500 or more cases per day.
Among 2,449 patients with known age, six-percent were aged ≥85, 25-percent were aged 65–84 years, 18-percent each were aged 55–64 years and 45–54 years, and 29-percent were aged 20–44 years. Only five-percent of cases occurred in persons aged 0–19 years.
Among 508 (12-percent) patients known to have been hospitalized, nine-percent were aged ≥85 years, 26-percent were aged 65–84 years, 17-percent were aged 55–64 years, 18-percent were 45–54 years, and 20-percent were aged 20–44 years. Less than one-percent of hospitalizations were among persons aged ≤19 years.
The percentage of persons hospitalized increased with age, from 2%–3% among persons aged ≤9 years, to ≥31% among adults aged ≥85 years.
Let’s unpack that real fast…
That means, from the time of this report, researchers only knew the age of about 60-percent of those infected. Of those 60-percent…
– 146 were older than 85
– 612 were between 65 and 84
– 440 were between 54 and 65
– 440 were between 45 and 54
– 710 were between 20 and 44
– 122 were younger than 19
That means about 46-percent, 1130 people, of those with a known age were in the workforce. If we extrapolate that percentage out to today’s number of 10,442, that means about 4800 people who could be working, are (hopefully) not working because of COVID-19.
In three days, using this extrapolation, the number of working-aged people has increased FOUR fold. That is incredible.
Doing the same calculation with those who were hospitalized gets you 1,255 estimated people hospitalized. And 690 of those were between 20 and 54. That number has doubled in three days.
If it doubles again in three days, that is 2,500. Another three days? 5,000 Another three days? 10,000 people. Hospitalized by this. By the end of next week.
By next Friday, extrapolating out the working-class numbers above, using the same four-fold increase, and 307,200 people will have the virus.
With a two-percent fatality rate of those 307,200 estimated people, 6,144 would die. And those who would die, would within six weeks as the virus took its course..
And while I have discussed the dangers of extrapolation before, and while this is an exaggerated example, this is why social distancing is so important. It will help curb those numbers dramatically if we all do our part.
More from the CDC…
Among 44 cases with known outcome, 15 (34-percent) deaths were reported among adults aged ≥85 years, 20 (46-percent) among adults aged 65–84 years, and nine (20-percent) among adults aged 20–64 years. Case-fatality percentages increased with increasing age, from no deaths reported among persons aged ≤19 years to highest percentages among adults aged ≥85 years.
Might be tough to tell if your child is infected
Speaking of statistics, In a recent study from a children’s hospital in China, only 40-percent of kids who had COVID-19 registered a fever. Nearly 60-percent had a fever less than 100-degrees. And said fever only lasted three days.
Other symptoms were cough (only about 50-percent had one), throat inflammation (only about 45-percent had this), and an increased heart rate (about 40 percent had this).
About 15-percent of kids showed zero symptoms. That means some children can have it, pass it along, and get better, without even knowing they are/were ever sick.
In a different study, from the same hospital, I believe, showed that one child only had the virus in his stool for two weeks, but was otherwise asymptomatic.
Choloroquine trial begins… soonish
A handful of new clinical trials and studies are set to begin. One study began on March 5th, titled “Clinical Trial of Favipiravir Tablets Combine With Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia” is set to complete in June. That study is being conducted in China.
The authors of the study write:
This study is a multi-centered, three-armed, randomized, double-blinded, controlled study, namely, the oral trial drug favipiravir tablets plus chloroquine phosphatetablets tablets group (combined group), the oral trial drug favipiravir tablets group (pirovir group), and the oral placebo treatment group (control group). The total number of enrolled cases in this study was set at 150.
During the treatment, the clinical data of the subjects were collected, the changes of viral load and biochemical indicators were detected, and the outcome of the subjects was monitored.
The main indicators of efficacy include improvement or recovery of respiratory symptoms and viral nucleic acid shedding. The rate of progression to severe disease, duration of fever, peripheral blood index and improvement time of pulmonary imaging were the secondary indicators to evaluate the efficacy.
Statistical analysis was performed at the middle and final stages of the study to evaluate the efficacy and safety of favipiravir tablets combined with chloroquine phosphatetablets tablets in the treatment of novel coronavirus pneumonia.
The other study, titled, “Chloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting” is set to begin in May. Because trials are just starting, I couldn’t find data on any treatments that have already used this drug. The researchers behind the study note:
The study is a double-blind, randomized, placebo-controlled trial that will be conducted in health care settings. After obtaining fully informed consent, the investigator will recruit healthcare workers, or other individuals at significant risk who can be followed reliably for 5 months. 10,000 participants will be recruited and the investigator predict an average of 200 participants per site in 50 sites.
The participant will be randomized to receive either chloroquine or placebo (1:1 randomization). A loading dose of 10mg base/kg, followed by 150 mg daily (250mg chloroquine phosphate salt) which will be taken for 3 months or until they are diagnosed with COVID-19. Subsequent episodes of symptomatic respiratory illness, including symptomatic COVID-19, clinical outcomes, and asymptomatic infection with the virus causing COVID-19 will be recorded during the follow-up period.
But, don’t look for Chloroquine to be widely administered to patients any time soon. While it has been approved – if I understood correctly – for use on certain patients in specific instances, it has not been approved for widespread use.
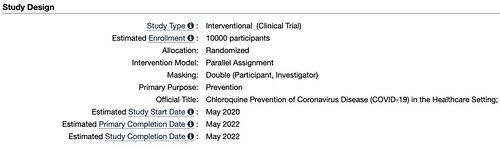
The clinical trial ends in May. Of 2022.
And, please – PLEASE! – do not sure this drug / chemical / etc without a prescription from a doctor.
A third study, titled, “Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19” started a week ago (roughly) and will end next year.
Notes from the authors of this study:
Phase 2:
The primary objective of the study is to evaluate the clinical efficacy of sarilumab relative to the control arm in adult patients hospitalized with severe COVID-19.
Phase 3:
The primary objective of the study is to evaluate the clinical efficacy of sarilumab relative to the control arm in adult patients hospitalized with severe or critical COVID-19.
And, I’ll alo say this about these three studies. And this is just an opinion. But while I am eager to get a vaccine and I am interested in finding a treatment. I am also interested in the doctors getting the science correct first. So the longer clinical trials are not a bad thing, in my opinion. It gives the scientists more time to determine how these drugs work, where they fail, and if there are any side effects.
From Harvard Medical
The Harvard Medical page has some Q&A on there occasionally. It looks like they got a great question that I’d like to share. It is along the same lines as the research discussed above.
Are chloroquine and hydroxychloroquine effective for treating COVID-19?
Recently, there has been considerable discussion of whether two related drugs — chloroquine and hydroxychloroquine — that have been available for decades to treat other illnesses might also be effective in treating COVID-19.
The drugs are primarily used to treat malaria and several inflammatory diseases, including systemic lupus erythematosus (lupus) and rheumatoid arthritis. No drug is perfectly safe, but these drugs are quite safe when used for just the several days they might be needed to treat COVID-19. They are also cheap, already available at our local drug stores, and relatively free of side effects.
The question, of course, is whether they are effective against the coronavirus that causes COVID-19. Are they effective in killing the virus in a laboratory dish? And are they effective in killing the virus in people? If the answer to the first question is “no,” there’s no point in getting an answer to the second question.
There is strong evidence that both drugs kill the COVID-19 virus in the laboratory dish. The drugs appear to work through two mechanisms. First, they make it harder for the virus to attach itself to the cell, inhibiting the virus from entering the cell and multiplying within it. Second, if the virus does manage to get inside the cell, the drugs kill it before it can multiply.
But do the drugs work in people with COVID-19? Many studies are underway to get an answer to this question, but as of March 24, 2020, only two have issued preliminary results.
One report, published in February 2020, claimed that chloroquine had been used in more than 100 patients in China who had COVID-19. The scientists stated that their results demonstrated that chloroquine is superior to the control treatment in inhibiting the worsening of pneumonia, improving lung imaging findings, eliminating the virus from the body, and shortening the duration of the disease.
These claims are exciting. However, the report provided virtually no evidence in support of the claims. First of all, this was not a randomized, double-blind controlled trial, the gold standard for research studies. Second, no evidence was presented as to how severe the pneumonia was, nor whether findings on lung x-rays or CT scans really improved. Third, although they claim the drug made the virus disappear, they didn’t report what the levels of the virus were before versus after the treatment. In short, not much evidence.
Another study is more encouraging. It was conducted by an excellent group of scientists in southern France, a region hard hit by COVID-19. This, also, was not a randomized trial. Instead, the scientists compared 26 patients who received hydroxychloroquine to 16 who did not: after six days, the virus was gone from the body in 70% of those given the treatment, compared to only 12.5% of those who weren’t. The drug appeared to be as effective in the sickest patients as in the least sick, but the study was too small to be sure about that. The study also was too small to say confidently that people who received the treatment were protected against a prolonged illness or death.
In summary, there is some reason for optimism that hydroxychloroquine may be effective in treating people with COVID-19. There are many studies underway, and we should have more solid answers within a few months.
On the other side of campus, Harvard is back to check on your mental well-being during all of this. Dr. Greg Fricchione, director of the Benson-Henry Mind Body Institute at Massachusetts General Hospital returns with another chat about how to handle all of the emotions that come along with Social Distancing.
Things you should be doing now
I know this can all seem overwhelming at times. The sheer amount of information being kicked out by every media outlets is like an avalanche.
So, here are some things: Stay home if you can! Gotta work? That’s understandable. Need food? Sure, head to the store. But try to skip any “for fun” activities in public where you would be interacting with others or in a place with multiple other people.
Some CDC’s guidance:
— Know where to get your local / state-level information
For Mississippi: https://msdh.ms.gov/msdhsite/_static/14,0,420.html
For Louisiana: http://ldh.la.gov/Coronavirus/
For Alabama: http://www.alabamapublichealth.gov/infectiousdiseases/2019-coronavirus.html
If you live in a state outside of the region, head to google and type in, “dept of health” followed by whatever state you live. Google should take you to that state’s department of health and on the main page, most states have a link to an update on the Coronavirus.
— Know the Symptoms
Look for things like a fever, dry cough, and shortness of breath. But also know when it may become an emergency. It becomes an emergency when you have difficulty breathing, a persistent pain or pressure in the chest, you develop general confusion, and if you develop bluish lips or face. Also recognize that body aches, weak stomach, nasal congestion, a sore throat, and other symptoms of the regular flu are not the same as the symptoms with Coronavirus.
— Stay home when you are sick
Any kind of sick. If you feel like you may have Coronavirus, call your health care provider’s office in advance of a visit. If you have any sickness, the CDC recommends to limit movement in the community, limit visitors, and practice good social distancing.
— Know if you are at a higher risk
Know what additional measures those at higher risk and who are vulnerable should take. Those at higher risk include older adults (over 60), people who have serious chronic medical conditions (like heart disease, diabetes, lung disease). Some research has indicated that people with asthma may also be included in the higher risk category.
— Take steps to mitigate your infection
The CDC recommends to “Implement steps to prevent illness” by washing high-traffic areas more often, washing hands with soap and water and if someone is sick, to isolate the sick person into a low-traffic area of the home.
— Create a Household Plan
Create a household plan of action in case of illness in the household or disruption of daily activities due to COVID-19 in the community.