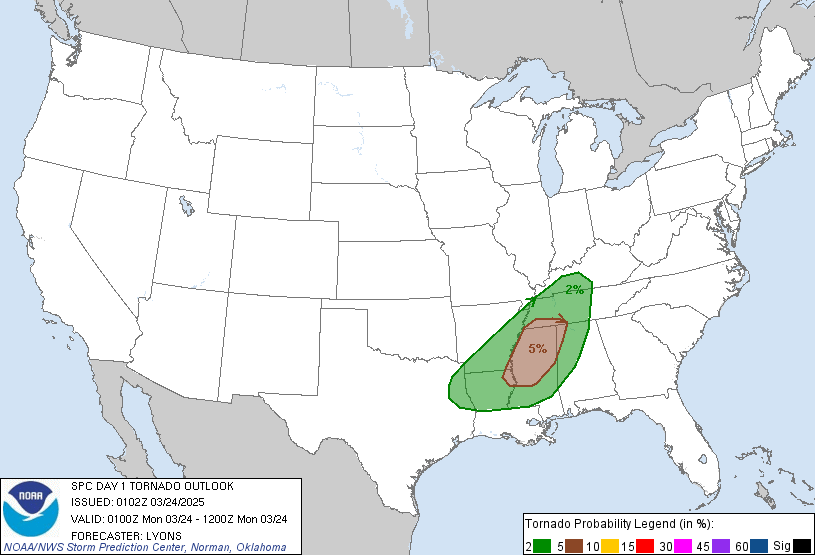
So, this is cool – literally and figuratively! This is the surface of a Birdbath, frozen over, with geometric shapes frozen into the surface.
I was asked, “what is happening here?”
And that is a great question!
To explain it, we have to go back to chemistry class. And we can do that while looking at snowflakes and rocks!
Recall that snowflakes form into a lot of crazy shapes. In fact, we’ve proven, mathematically, on the site that no two snowflakes can be the same. The reason they grow into different shapes has to do with how the water molecules form and bond to each other.

Water, like every other molecule, aligns in certain directions as it cools and freezes. Another great example of this in nature is from rocks. Have you ever noticed that some rocks have a very crystaline structure and others just look craggy and clumpy? There is a reason!
Part of the reason is about how fast the rocks cool. The same is true for snowflakes. And water!
I had a chance to meet up with a former geochemistry professor of mine, on the campus of Oregon State University, and ask him about his take on what was happening. This was his explanation:
“When cool things slower, the crystalline structure can align easier,” Dr. Adam Kent, Professor and Associate Dean for Graduate Programs and Faculty Advancement, said (he has had a bit of a promotion since I left school). “But if you cool thing in a flash, they tend to be smoother because the molecules can’t align to give you any kind of structure. Like that glass there (he said pointing toward a window) formed because it went from molten to frozen very quickly.”
And the same is true for regular water.
If you slowly cool down water and slowly freeze it, it allows all of the molecules to align in a more crystalline pattern. And you get this:

Because water, once it is frozen, is really no different than a rock.